Diagnosis
Multiple Myeloma
Introduction
A 38 year old female with past medical history of mental retardation and asthma presents to the emergency department complaining of worsening left shoulder pain for two days associated with headache, dizziness, nausea and non-bloody non-bilious vomiting. Patient’s vital signs were normal, and exam was only remarkable for mild obesity, pallor of the skin and conjunctiva, and tenderness to palpation over the left upper arm with decrease active range of motion due to pain. There was no history of trauma.
Hospital Course
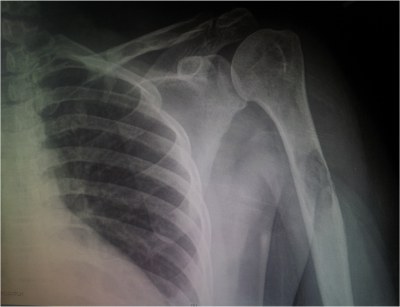
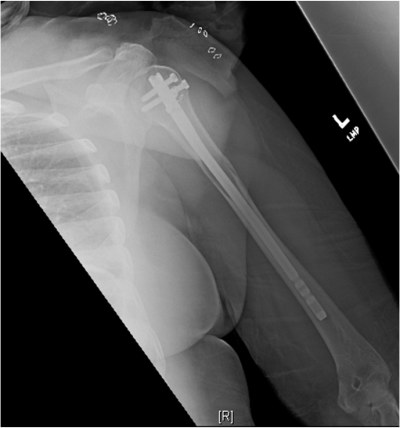
Patient received pain medication and symptomatic treatment. X-ray of the left shoulder showed an expansile lesion within the proximal humeral diaphysis [fig. 2] compromising the bone integrity and increasing the risk of pathological fracture. The differential diagnosis included benign and malignant lesions of the bone, including primary or secondary malignancies and hematologic malignancies. After the diagnosis was confirmed, patient underwent prophylactic fixation of the left humerus with an intramedullary rod. [fig. 3]
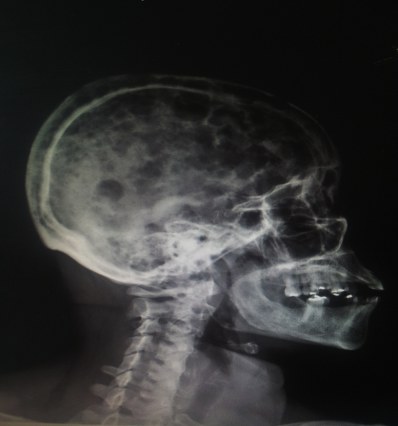
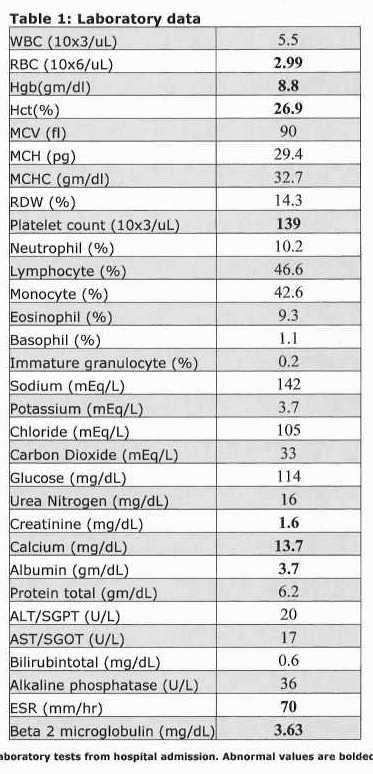
Further work up revealed hypercalcemia, renal failure and anemia. Erythrocyte sedimentation rate was 70 mm/hr and beta2 microglobulin level was 3.63 mg/dL. [fig. 1] Patient tested negative for HIV, hepatitis C antibodies and was immune to hepatitis B. Skeletal survey showed multiple lytic lesions involving the skull [fig. 4] , right seventh posterior rib, right and left humerus.
Serum immunofixation showed no monoclonal proteins, but decreased levels of IgG, IgA and IgM. Serum free light chains showed an excess of kappa light chains measuring 590 mg/dL. Urine protein electrophoresis and immunofixation showed a Bence-Jones proteinuria with free kappa light chains.
Bone marrow aspiration and biopsy showed extensive infiltration by plasma cells with lymphoplasmacytoid morphology. Flow cytometry revealed 48% IgG kappa restricted plasma cells. Bone marrow cytogenetics showed an abnormal karyotype, with thirteen mitotic cells characterized by loss of one copy of chromosome 13 and the X chromosome, a deletion in the short arm of chromosome 1, a translocation between the long arms of chromosomes 11 and 14, a derivative homologue chromosome 14 resulting from the t(11;4), and 0~11 double minutes. The remaining 7 cells had no chromosomal abnormalities. Fluorescence in situ hybridization revealed t(11;14) / cyclin D1-IGH translocation and also monosomy 13 in 96% of the cells.
Overall, the patient was considered to have stage IIIB multiple myeloma (per Durie-Salmon staging system) with an unfavorable prognosis disease and high burden of disease. Giving these factors and the patient’s age, she was felt to be a good candidate for curative intent therapy. She was referred to a tertiary care center for upfront high dose chemotherapy with possible autologous stem cell transplantation.
After once cycle of treatment, she has tolerated chemotherapy well although she developed grade I mucositis, peripheral neuropathy and developed culture negative neutropenic fever.
Discussion
Multiple myeloma (MM) is a malignant disorder, which is characterized by neoplastic proliferation of plasma cells in the bone marrow, producing monoclonal immunoglobulins and associated organ dysfunction. Bone marrow infiltration by malignant plasma cells results in extensive skeletal destruction with osteolytic lesions, osteopenia, bone pain, and/or pathological fractures. Multiple myeloma accounts for approximately 1% of all cancers and about 10 percent of hematologic malignancies in the United States. 1
MM is a disease of the older adult. The median age at diagnosis is approximately 70 years. 2 Less than 2% of patients are younger than 40 years of age at diagnosis and this disease is extremely rare in those younger than 30 years, with a frequency in patients younger than 40 and 30 years of 2.2% and 0.3%, respectively. 3 The main presenting clinical features are bone pain (66%), fatigue (26%), extramedullary plasmacytomas (19%) and bacterial infection (11%), renal function impairment (Creatinine level ≥ 177μmol/l) and hypercalcemia (serum calcium value ≥2.75 mmol/l) occurs in 29% and 30% of patients, respectively. 4
The International Myeloma Working Group criteria for the diagnosis of MM emphasize the importance of end organ damage in making the diagnosis. The following criteria are required for the diagnosis of MM: bone marrow clonal plasma cells ≥10 percent or biopsy proven bony or soft tissue plasmacytoma and one of the following: organ/tissue damage evidenced by increased Calcium level, Renal insufficiency, Anemia, Bone lesions (recalled by the acronym CRAB); or presence of one or more biomarkers of malignancy: bone marrow plasma cell ≥60%, serum free light chain ratio of 100 or ≥100mg/L and/or >1 focal lesions on MRI studies. 5
Multiple myeloma may be staged by using the Durie-Salmon system. Although this system is still used, its value is becoming limited because of newer diagnostic methods. Recently, a new staging system called the International Staging System for MM has been developed. It relies mainly on levels of albumin and beta-2-microglobulin in the blood. Other factors that may be important are kidney function, platelet count and the patient’s age. 6
The International Staging System (ISS) divides MM into 3 stages based only on the serum beta-2 microglobulin and serum albumin levels: stage I has a beta-2 microglobulin levels of less than 3.5 mg/L with an albumin greater than 3.5 g/dl, stage II is neither stage I or III, while stage III has a beta-2 microglobulin level of greater than 5.5 mg/L. 6
Based on this staging system our patient has stage II MM. Age is also important and in studies using staging according to the ISS, older people with myeloma do not live as long. The clinical behavior of multiple myeloma in adolescents and young adults has been shown to be more indolent. 7 8
There are at least 5 classes of active agents for initial therapy of MM: alkylating agents, anthracyclines, corticosteroids, immunomodulatory drugs and proteasome inhibitors. High-dose chemotherapy with autologous stem cell support (ASCT) remains the standard of care in transplant eligible patients, based on a series of randomized trials that found improved progression free survival and overall survival. Patients eligible to undergo ASCT generally receive 4 cycles of induction chemotherapy prior to stem cell collection to reduce the number of tumor, treat symptoms and reduce end-organ damage. Patients ineligible for ASCT, mostly patients older than 70 years or with significant co-morbid illness, will not be treated with high-dose therapy and stem cell support. This type of patients can receive induction chemotherapy generally until progression of disease unless patient develops severe toxicity. Owing to the risk of adverse events in elderly patients, combined with the prolonged survival now seen with newer therapies, balancing efficacy and toxicity is particularly important in this group. 9
Figures
References
-
Siegel R, Miller K, Jemal A. Cancer Statistics, 2015. CA: A Cancer Journal for Clinicians 2015; 65: 5–29. Retrieved from http://onlinelibrary.wiley.com/doi/10.3322/caac.v65.1/issuetoc
-
Palumbo A, Anderson K. Multiple Myeloma. New England Journal of Medicine 2011; 364: 1046–1060. Retrieved from http://www.nejm.org/doi/full/10.1056/NEJMra1011442
-
Bladé J, Kyle RA, Greipp PR. Multiple Myeloma in Patients Younger than 30 Years: Report of 10 Cases and Review of the Literature. Archives of Internal Medicine 1996; 156: 1463–1468. Retrieved from http://archinte.jamanetwork.com/article.aspx?articleid=622168
-
Bladé J, Kyle RA, Greipp PR. Presenting Features and Prognosis in 72 Patients With Multiple Myeloma Who Were Younger Than 40 Years. British Journal of Haematology 1996; 93: 345–351. Retrieved from http://onlinelibrary.wiley.com/doi/10.1046/j.1365-2141.1996.5191061.x/abstract
-
Rajkumar SV, Dimopoulos MA, Palumbo A, Bladé J, Merlini G, Mateos MV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 2014; 15:538-548. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/25439696
-
Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Bladé J, et al.International staging system for multiple myeloma. J Clin Oncol 2005; 23:3412-3420. Retreived from http://www.ncbi.nlm.nih.gov/pubmed/15809451
-
Rapoport AP, Rowe JM. Plasma Cell Dyscrasia in a 15-Year-Old Boy: Case Report and Review of the Literature. The American Journal of Medicine 1990; 89: 816–818. Retrieved from http://www.amjmed.com/article/0002-9343(90)90229-7/abstract
-
Lazarus HM, Kellermeyer RW, Aikawa M, Herzig RH. Multiple Myeloma in Young Men Clinical Course and Electron Microscopic Studies of Bone Marrow Plasma Cells. Cancer 1980; 46: 1397–1400. Retrieved from http://onlinelibrary.wiley.com/doi/10.1002/1097-0142(1980091546:6%3C1397::AID-CNCR2820460618%3E3.0.CO;2-F/abstract
-
Mikhael JR, Dingli D, Roy V, Reeder CB, Buadi F, Hayman SR, et al.Management of Newly Diagnosed Symptomatic Multiple Myeloma: Updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) Consensus Guidelines 2013. Mayo Clinic Proceedings 2013; 88: 360–376. Retrieved from http://www.mayoclinicproceedings.org/article/S0025-6196(13)00077-3/fulltext#sec9