INTRODUCTION
Coronary intervention is an invasive procedure associated with short and long-term complications. However, at the same, it is lifesaving and significantly reduces the morbidity and mortality of patients if done promptly. It is worthwhile to explore different strategies to reduce the significant complication associated with coronary angiography. This case signifies Innovative technology in coronary angiography to minimize complications in high-risk patients.
Case Report
An 86-year-old Hispanic female with Hypertension, Coronary artery disease (CAD), Peripheral Arterial Disease (PAD) s/p left Superficial Femoral Artery (SFA) stent, Chronic Kidney Disease (CKD) stage 3, Chronic Obstructive Pulmonary Disease (COPD) on home-oxygen, colonic cancer s/p hemicolectomy and colostomy presented with sudden onset dyspnea. She was admitted with hypertensive emergency with acute heart failure and Non-ST elevation Myocardial Infarction (NSTEMI). The examination was significant for a Heart rate of 145 bpm, Blood pressure of 193/103 mmHg, RR of 22 breaths/min, temperature 98.2-degree Fahrenheit, elevated JVP, bilateral lung crackles.
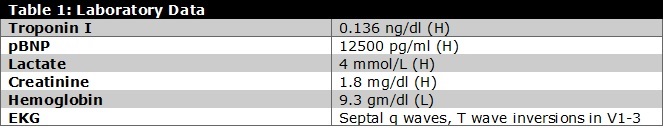
*Note: (H) indicates high. (L) indicates low.
Further care: The patient was stabilized with nitroglycerine drip and furosemide. She was also given clopidogrel, aspirin, and a heparin drip, but with rising troponin of 8.5 ng/dl, she was referred for coronary angiography. Echocardiogram showed Ejection fraction (EF) of 25%, diffuse hypokinesis with distinct regional variations. Initial catheterization revealed 99% proximal to mid LAD in-stent restenosis, 100% distal circumflex thrombotic occlusion, 90% mid RCA stenosis. [Figure 1] Patient became hypotensive during the procedure and right heart catheterization confirmed cardiac output (thermodilution) 2.5 L/min, cardiac index 1.64 L/min/m2, Right Atrial Pressure (RAP) 7 mmHg, mean Pulmonary artery pressure (PAP) 29 mmHg, Pulmonary Capillary Wedge Pressure (PCWP) 28 mmHg. Surgery was consulted and deemed a prohibitive risk for Coronary Artery Bypass Graft (CABG). Hence, the patient underwent successful Percutaneous coronary intervention (PCI) of Left anterior descending artery (LAD) with Drug Eluting Stent (DES 3.0mm x 38mm) and circumflex lesions (DES 2.75mm x 26mm).[Figure 2] An intra-aortic-balloon pump was placed with pressor support, and a staged intervention of RCA was planned.
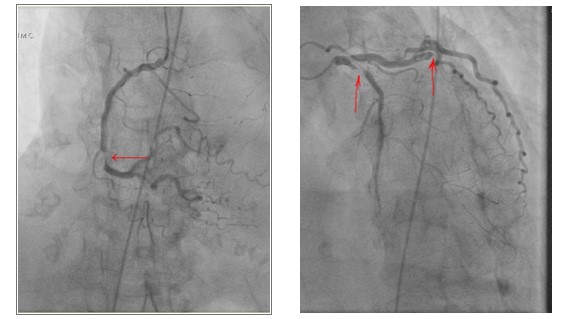
Figure 1: Coronary angiogram revealed 99% proximal to mid LAD in-stent stenosis, 100% distal circumflex thrombotic occlusion, 90% mid RCA stenosis.
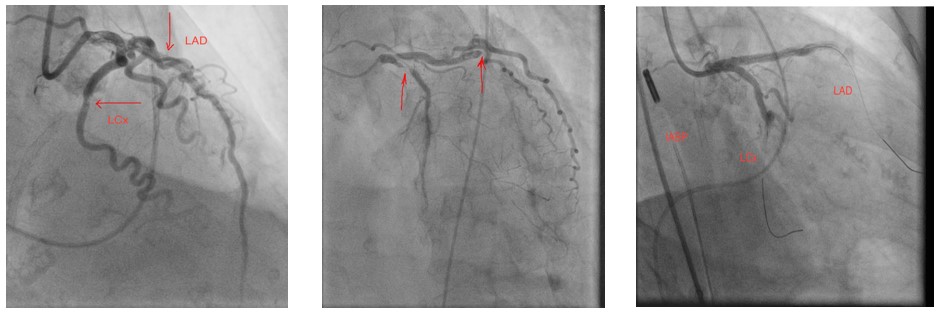
Figure 2: A zotarolimus drug eluding stent (Resolute Onyx), 3.0mm x 38mm and 2.75mm x 26mm were deployed in proximal LAD and distal circumflex (LCx) respectively
Interventional Management: The patient was successfully weaned off pressor/intra-aortic balloon-pump after 48hrs, but recovery was complicated by contrast-induced nephropathy (CIN) with Cr 5.2 hence staged RCA was deferred. On day eight patient underwent successful PCI without contrast with placement of DES 3.0mm x 15mm.[Figure 3] RCA catheter engagement was confirmed by transient T wave repolarization changes following 5cc of cold saline guide catheter infusion; stent size and length was determined using IVUS to define the lesion.[Figure 4] On day 17, the renal function returned to baseline without dialysis and was discharged on guideline-directed medical therapy to rehab with improved EF to 45-50% on repeat echocardiogram.

Figure 3: Prior diagnostic image showing mid RCA lesion, beside is a similar image with advancement of coronary wire beyond the lesion and a subsequent image with stent deployed.
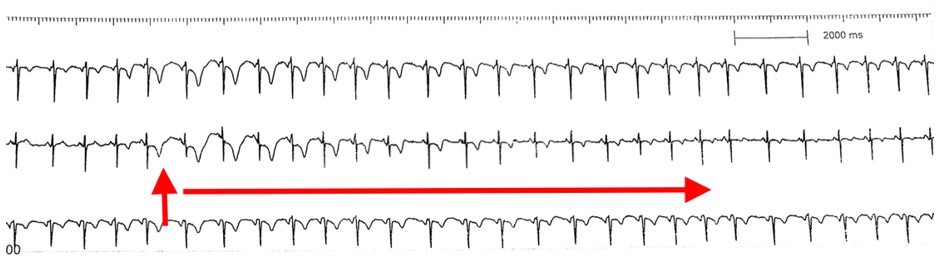
Figure 4: Rhythm strip showing sudden transient T wave changes during cold saline injection into the RCA confirmed vessel engagement
INTRODUCTION
The incidence of contrast-induced nephropathy during PCI can range up to 5%, with a much higher incidence in patients over age 45 years, EF less than 45%, and baseline renal disease. 1 Usually, the incidence can be as low as 3% in the general population but rises significantly in higher risk patients. 2 Contrast-induced nephropathy (CIN) refers to an increase in the creatinine by 25% or an increase in absolute value by more than 44.2 micro moles/L after contrast administration. 3 Pathophysiology of contrast-induced nephropathy could be secondary to a combination of decreased renal medullary blood flow resulting in medullary ischemia, free radical formation, and direct toxic effect on tubular cells. 4 Risk factors noted in prior studies include preexisting renal disease, diabetes, congestive heart failure, elevated NT-proBNP level, dehydration, anemia, hypertriglyceridemia, hyperuricemia, blood loss, and use of nephrotoxic drugs. 5 Intra-arterial injection of contrast is much more damaging to kidneys than intravenous contrast. 6 In the case of intravenous injection, it takes longer to travel to the kidneys as compared to intra-arterial during coronary angiography. Angioplasty in the emergency setting has a much higher incidence of CIN, ranging from 15-30% with higher in-hospital mortality. After two years, the mortality with contrast-induced nephropathy leading to dialysis can be as high as 40%. 7 The use of furosemide with hydration has shown promising effects in preventing CIN compared to hydration alone. 8 The use of peri procedure hydration is the accepted method to prevent nephropathy. 9 , 10 Administration of sodium bicarbonate has not shown superiority over isotonic saline to prevent contrast-induced nephropathy. Hydration through volume expansion, leading to a reduction in renal vasoconstriction and reducing the urine viscosity and lesser contrast, stay in the tubules, helps prevent the contrast induced nephropathy. In an emergent setting, the above preventive measures cannot be taken, increasing the incidence of this complication. In patients with depressed left ventricular function, excessive intravenous hydration cannot be done. Given all this, the contract-free strategy will be a unique technique in avoiding this complication. Intra-coronary cold saline can cause alteration in ventricular repolarization with transient ECG changes to guide correct catheter placement and insertion of devices. Lesion length for appropriate stent sizing can be ascertained by intravascular ultrasound. In this way, percutaneous intervention can be done in patients with renal failure without any contrast agents.
CONCLUSION
CIN further complicate intervention in high-risk PCI, contrast-free strategy helped to drastically mitigate this risk in our patient and achieve complete revascularization with excellent result. This was an extremely high-risk triple vessel CAD not amenable to surgery. It was further complicated by cardiogenic shock and acute renal failure with potentially fatal outcomes. Despite these risks, complete revascularization was achieved with the recovery of Left Ventricular function and preservation of renal function using contrast-free staged PCI.
Statement of Authorship
All authors have given approval to the final version submitted.
Author Disclosure
All the authors have declared no conflict of interest to the work carried out in this paper.
Funding Source
None
REFERENCES
-
Wang J, Zhang C, Liu Z, Bai Y. Risk factors of contrast-induced nephropathy after percutaneous coronary intervention: a retrospective analysis. J Int Med Res. 2021; 49
-
Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002; 105: 2259-64.
-
Hong WY, Kabach M, Feldman G, Jovin IS. Intravenous fluids for the prevention of contrast-induced
nephropathy in patients undergoing coronary angiography and cardiac catheterization. Expert Rev Cardiovasc Ther. 2020; 1833-39. -
Persson PB, Hansell P, Liss P. Pathophysiology of contrast medium-induced nephropathy. Kidney Int. 2005; 68:14-22.
-
Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, et al. A simple risk score for prediction of contrast induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004; 44:1393-9.
-
Katzberg RW, Barrett BJ. Risk of iodinated contrast material–induced nephropathy with intravenous
administration. Radiology. 2007 Jun; 3:622-8. -
McCullough PA, Soman SS. Contrast-induced nephropathy. Crit Care Clin. 2005; 21:261-80.
-
Marenzi G, Ferrari C, Marana I, Assanelli E, De Metrio M, Teruzzi G, et al. Prevention of contrast
nephropathy by furosemide with matched hydration: the MYTHOS (Induced Diuresis with Matched Hydration Compared to Standard Hydration for Contrast Induced Nephropathy Prevention) trial. JACC Cardiovasc Interv. 2012; 5:90-7. -
Mueller C, Buerkle G, Buettner HJ, Petersen J, Perruchoud AP, Eriksson U, et al. Prevention of contrast media-associated nephropathy: randomized comparison of 2 hydration regimens in 1620 patients undergoing coronary angioplasty. Arch Intern Med. 2002; 162:329-36.
-
Luo Y, Wang X, Ye Z, Lai Y, Yao Y, Li J, et al. Remedial hydration reduces the incidence of contrast-induced nephropathy and short-term adverse events in patients with STsegment elevation myocardial infarction: a single-center, randomized trial. Intern Med. 2014; 53:2265-72.
